Binciken bayanan jama'a
Data on SARS-CoV-2 lineages and the date the sample was taken from the sequences available from Chile were obtained from the Consorcio Genomas CoV2 site available at https://auspice.cov2.cl/ncov/chile-global. Vaccination data were obtained from public data from Ministry of Science, Technology, Knowledge and Innovation available at https://github.com/MinCiencia/Datos-COVID19 (Product 83).
Gwajin kamuwa da cuta
Pseudotyped viruses carrying different SARS-CoV-2 spike proteins were prepared as we previously described12. Briefly, HIV-1-based SARS-CoV-2 pseudotypes were produced in HEK293T cells by transfecting the pNL4.3-ΔEnv-Luc together with the corresponding pCDNA-SARS-CoV-2 Spike coding vector in a 1:1 molar ratio. Plasmids coding a codon-optimized spike lacking the last 19 amino acids of the C-terminal end (SΔ19) known to avoid retention at the endoplasmic reticulum12 were obtained by gene synthesis or customized site-directed mutagenesis (GeneScript) and contained the following mutations: lineage A (reference sequence), lineage B (D614G), lineage B.1.1.7 (Δ69-70, Δ144, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H), lineage P.1 (L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I) and lineage C.37 (G75V, T76I, Δ246-252, L452Q, F490S, D614G, T859N). Each pseudotype preparation was cleared by centrifugation at 3,000 rpm at room temperature, quantified using the HIV-1 Gag p24 Quantikine ELISA Kit (R&D Systems), aliquoted in 50% fetal bovine serum (Sigma-Aldrich) and stored at -80 °C until use. Different amounts of pseudotyped viruses (as determined by the levels of the HIV-1 p24 protein) were used to infect HEK-ACE2 cells and 48 hours later, firefly luciferase activity was measured using the Luciferase Assay Reagent (Promega) in a Glomax 96 Microplate luminometer (Promega).
Gwajin Neutralization
Pseudotyped virus neutralization assays were performed essentially as we previously described12. Briefly, serial dilutions of the plasma samples (1:4 to 1:8748) were prepared in DMEM with 10% fetal bovine serum and incubated with 5 ng of p24 of each pseudotyped virus during 1h at 37 °C and then, 1×104 of HEK-ACE2 cells were added to each well. HEK293T cells (not expressing ACE2) incubated with the pseudotyped virus (lineage A) were used as a negative control. Cells were lysed 48 hours later, and firefly luciferase activity was measured using the Luciferase Assay Reagent (Promega) in a Glomax 96 Microplate luminometer (Promega). The percentage of neutralization for each dilution was calculated and the ID50 of each sample was calculated using GraphPad Prism version 9.0.1.
Nazarin ilimin lissafi
Statistical analyses were performed using GraphPad Prism software version 9.1.2. Multiple group comparisons for neutralizing antibody titers (NAbTs) against a panel of SARS-CoV-2 pseudotyped viruses as well as comparisons of NAbs responses by sex and smoke status were carried out using a paired Wilcoxon signed-ranked test. Factor change was calculated as the difference of geometric mean titer in the ID50 as compared with that of the Wild type pseudotyped virus. Correlation analysis between NAbTs and age or BMI was carried out using Spearman’s test. One-way ANOVA and Tukey’s multiple comparison test were performed for statistical analysis of infectivity. A p value ≤0.05 was considered as statistically significant.
Amincewa da dabi'a
The study protocol was approved by the Ethics Committee of the Faculty of Medicine at Universidad de Chile (Projects N° 0361-2021 and N° 096-2020) and Clínica Santa Maria (Project N°132604-21). All donors signed the informed consent, and their samples were anonymized.
Tasirin sauye sauye a cikin bambance -bambancen Lambda akan kamuwa da cuta da kawar da martani na ƙwayoyin cuta
An analysis of 3695 sequences from Chile deposited at GISAID as per June 24th 2021 shows a clear dominance of the SARS-CoV-2 variants Gamma and Lambda during the last trimester accounting, together, for the 79% of all sequences.
Interestingly, this period has been characterized by a massive vaccination campaign in which 65.6% of the target population (18 years old and older individuals) have received a complete vaccination scheme as per June 27th 2021
Given that 78.2% of the people inoculated with a complete scheme received the inactivated virus vaccine CoronaVac from Sinovac Biotech, we sought to investigate the impact of the spike mutations present in the Lambda variant on the neutralizing capacity of antibodies elicited by this vaccine.
For this, we generated HIV-1-based SARS-CoV-2 pseudotyped viruses carrying the spike protein from the Wuhan-1 reference lineage (Wild type; lineage A), the D614G mutation (lineage B), and the Alpha (lineage B.1.1.7), Gamma (lineage P.1) and Lambda (lineage C.37) variants.
During virus preparation, we consistently observed that cells infected with the pseudotyped virus carrying the Lambda spike produced significant higher bioluminescence values when compared to the D614G mutant or the Alpha and Gamma variants indicating increased infectivity driven by the Lambda spike protein

Hoto 1.Infectivity mediated by different spike proteins.
(A) Schematic representation of the SARS-CoV-2 spike protein and the variants used in this study. Lineages are indicated in parenthesis. RBD, receptor-binding domain, CM; cytoplasmic tail.
(B) Titration of the pseudotypes of each lineage using equivalent amounts of HIV-1 p24. The firefly luciferase activity was measured as relative luminescence units (RLU) at 48 hours post-infection. The average and SD were calculated from a representative triplicate experiment.
Next, we used the pseudotyped viruses mentioned above to performed neutralization assays using 79 plasma samples from healthy healthcare workers from Universidad de Chile and Clínica Santa María at Santiago, Chile.
We excluded 4 samples as we were not able to calculate an ID50 titer. From the analyzed samples, 73% corresponded to women, median age 34 years (IQR 29 – 43) and a body max index (BMI) of 25 (IQR 22.7 – 27). A 20.5% of the participants declared to be active smokers while the immunization period lasted. Samples were obtained in a median of 95 days (IQR 76 – 96) after the second dose of the CoronaVac vaccine
We observed that neutralization of the pseudotyped virus carrying the Wild type spike protein resulted in a 50% inhibitory dilution (ID50) mean titer of 191.46 (154.9 – 227.95, 95% CI,), while it was 153.92 (115.68 – 192.16, 95% CI), 124.73 (86.2 – 163.2, 95% CI), 104.57 (75.02 – 134.11, 95% CI) and 78.75 (49.8 – 107.6, 95% CI) for pseudotyped viruses carrying the spike protein from the D614G mutant or the Alpha, Gamma and Lambda variants, respectively.
We also observed that the geometric mean titer of the ID50 titers decreased by a factor of 3.05 (2.57 – 3.61, 95% CI) for the pseudotyped virus carrying the Lambda spike, 2.33 (1.95 – 2.80, 95% CI) for the Gamma spike, 2.03 (1.71 – 2.41, 95% CI) for the Alpha spike and 1.37 (1.20 – 1.55, 95% CI) for the D614G spike when compared to the Wild type spike.
No correlation between sex, age, body-mass index (BMI) or smoke status and neutralizing antibody titers were observed in our study cohort.
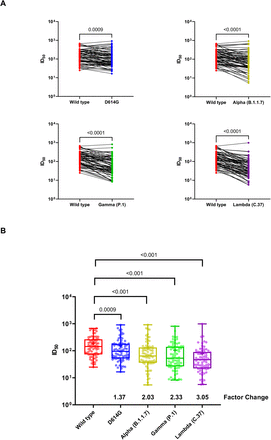
Hoto 2.Neutralization assay using plasma samples from CoronaVac vaccinees
(A) Changes in the reciprocal 50% neutralization titer (ID50) in plasma samples from 75 recipients of the CoronaVac vaccine against the D614G (lineage B), Alpha (lineage B.1.1.7),
Gamma (lineage P.1) and Lambda (lineage C.37) variants as compared with wild-type virus. Results are shown as the difference in neutralization titers of matched samples. P values for the comparison of the ID50 are calculated with Wilcoxon signed-rank test.
(B) Box plots indicated the median and interquartile range (IQR) of ID50 for each pseudotyped virus. Factor changes are shown as the difference of geometric mean titer in the ID50 as compared with those for the Wild type pseudotyped virus. Statistical analyses were performed using the Wilcoxon matched-pairs signed-rank test.
Together, our data revealed that the spike protein of the newly recognized variant of interest Lambda, highly circulating in Chile and South American countries, carries mutations conferring increased infectivity and the ability to escape from neutralizing antibodies elicited by CoronaVac.
ABUBUWAN DA ZA KU GUDU DAGA WANNAN LABARI:
- An analysis of 3695 sequences from Chile deposited at GISAID as per June 24th 2021 shows a clear dominance of the SARS-CoV-2 variants Gamma and Lambda during the last trimester accounting, together, for the 79% of all sequences.
- Different amounts of pseudotyped viruses (as determined by the levels of the HIV-1 p24 protein) were used to infect HEK-ACE2 cells and 48 hours later, firefly luciferase activity was measured using the Luciferase Assay Reagent (Promega) in a Glomax 96 Microplate luminometer (Promega).
- 2% of the people inoculated with a complete scheme received the inactivated virus vaccine CoronaVac from Sinovac Biotech, we sought to investigate the impact of the spike mutations present in the Lambda variant on the neutralizing capacity of antibodies elicited by this vaccine.